[無料ダウンロード! √] yield formula chemical engineering 135422-Fractional yield formula chemical engineering
High Yield Question Bank GATE 2122 Chemical Engineering GATE ACADEMY CE ME CH XE PI Telegram Channel Gate academy official Online Test Series What is the natural period of oscillation (in min) of the system?C Note that the actual ratio of smaller than the required or stoichiometric ratio, which means there is insufficient H 2 to react with all of the O 2 that has been provided The 'insufficient' component (H 2) is the limiting reactantAnother way to put it is to say that O 2 is in excess When the reaction has proceeded to completion, all of the H 2 will have been consumed, leaving some O 2Course Description LearnChemE features faculty prepared engineering education resources for students and instructors produced by the Department of Chemical and Biological Engineering at the University of Colorado Boulder and funded by the National Science Foundation, Shell, and the Engineering Excellence Fund
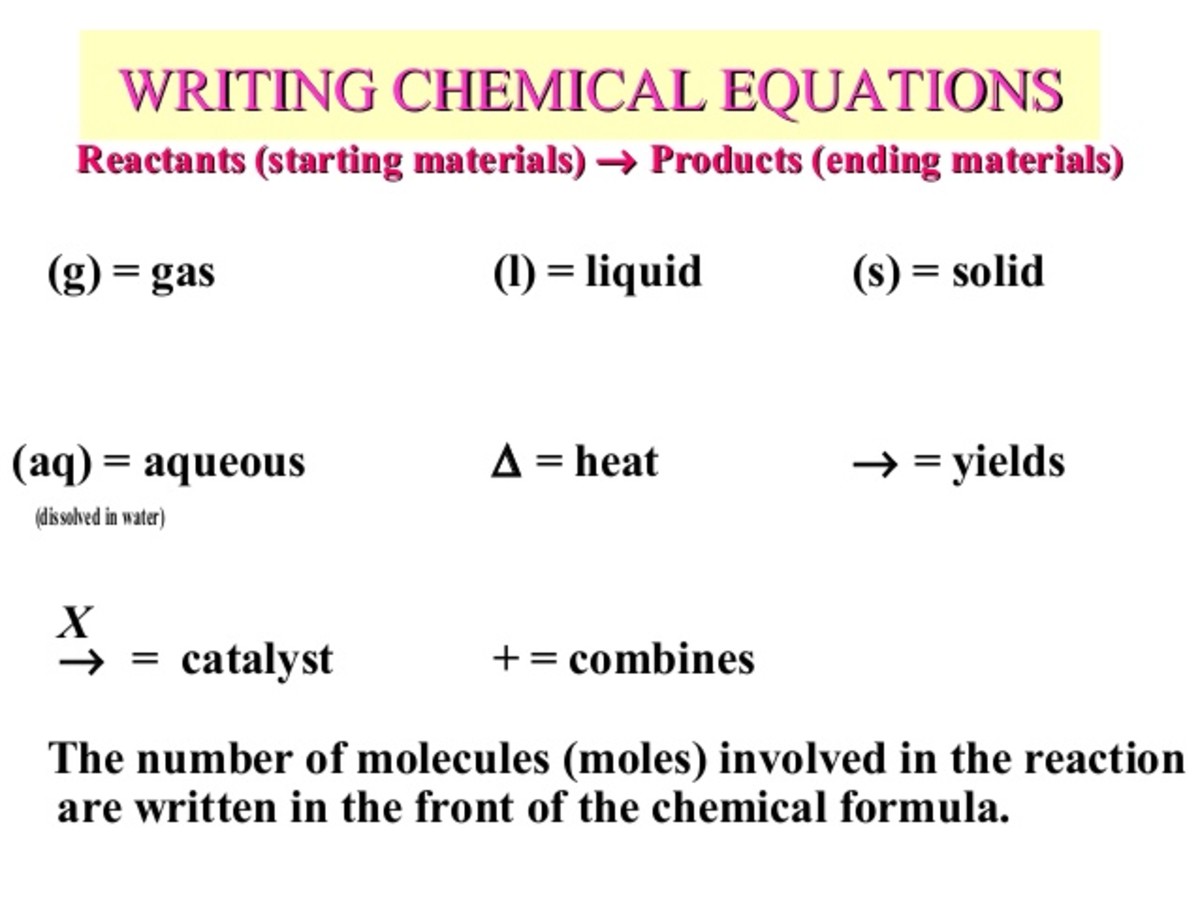
Chemical Reactions And Chemical Equations Owlcation Education
Fractional yield formula chemical engineering
Fractional yield formula chemical engineering-Chemical Analysis Lists the content values of various elements expressed as a share of one percent (ex 30 of carbon=003) Tensile Strength Also called ultimate strength, measurement at which steel exhibits strain Yield Strength Related to tensile, yield is the stress level at which steel exhibits strainThe percentage yield is obtained by multiplying the fractional yield by 100% One or more reactants in a chemical reaction are often used in excess and thus the theoretical yield is therefore calculated based on the molar amount of the limiting reactants, keeping in mind the stoichiometry of the reaction


Q Tbn And9gctgqe Sq69j9qphav8vjnq7bdheoratbvanabkki0b6yruaya Usqp Cau
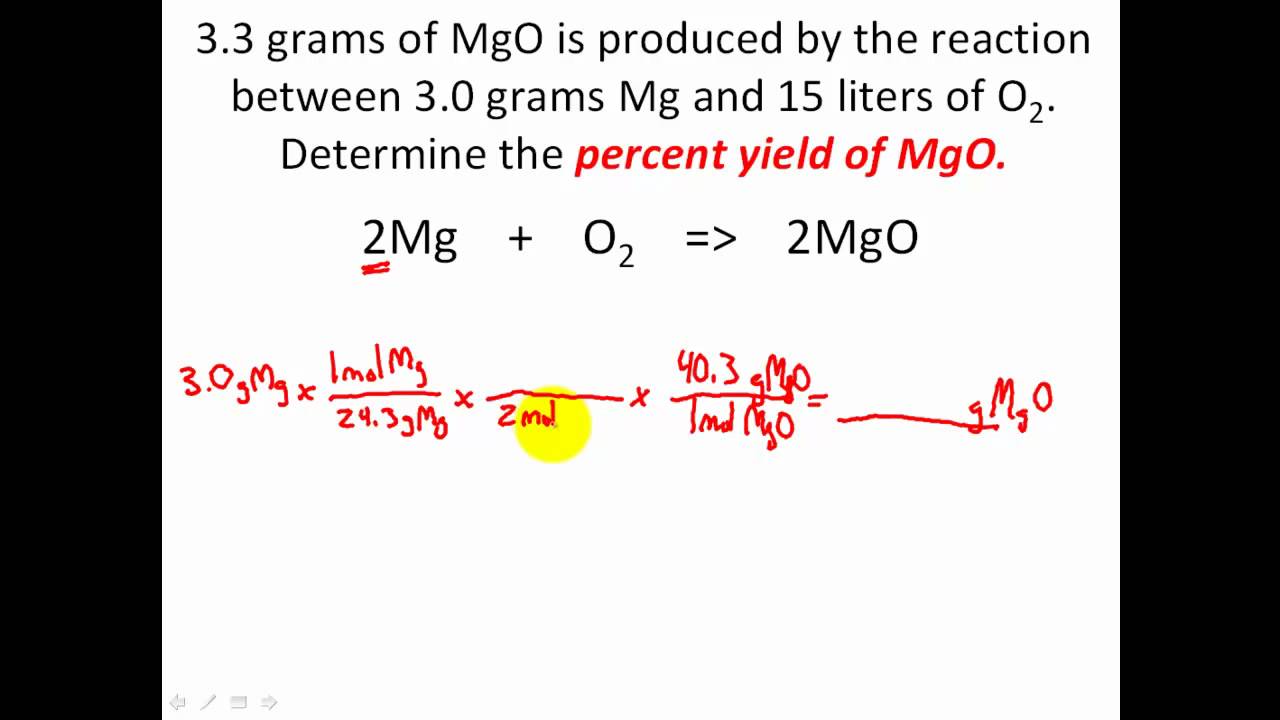
2 What is the first thing we need to check when using a chemical equation?Usually, you have to calculate the theoretical yield based on the balanced equation In this equation, the reactant and the product have a 11 mole ratio, so if you know the amount of reactant, you know the theoretical yield is the same value in moles (not grams!)You take the number of grams of reactant you have, convert it to moles, and then use this number of moles to find out how many142a Percentage yield of the product of a reaction Even though no atoms are gained or lost in a chemical reaction (law of conservation of mass), unfortunately it is not always possible to obtain the calculated amount of a product (ie 100% yield) because the reaction may not go to completion because it may be reversible or some of the product may be lost when it is separated from the
Course Description LearnChemE features faculty prepared engineering education resources for students and instructors produced by the Department of Chemical and Biological Engineering at the University of Colorado Boulder and funded by the National Science Foundation, Shell, and the Engineering Excellence FundYield definition If you yield to someone or something, you stop resisting them Meaning, pronunciation, translations and examplesAs the chemical engineering community is collecting more data (volume) from different sources (variety), this journey becomes more challenging in terms of using the right data and the right tools
C 7 H 16 11 O 2 7 CO 2 8 H 2 O 1 What information can we get from this equation?This article is cited by 43 publications V Chandra, EAJF Peters, JAM Kuipers Direct numerical simulation of a nonisothermal nonadiabatic packed bed reactorThis simple estimation will often yield a result greatly under a value needed for a good cost estimations because in the modern financial economy, cash deposits yield minimal interest rates WACC is described by the following formula 3 where is the number of sources of capital Chemical Engineering Design Principles, Practice and
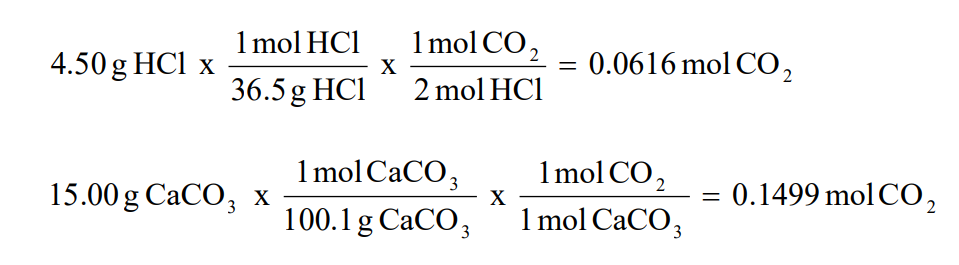


Molecular Formulas And Nomenclature



Howto How To Find Percent Yield Without Actual Yield
2 What is the first thing we need to check when using a chemical equation?Biochemical Engineering has been ofiered as one of the elective courses to the Universiti Sains Malaysia's Chemical Engineering undergraduates since 1998 under the topic of Bioprocess Engineering The change of name from Bioprocess to Biochemical Engineering shows that the School of Chemical Engineering is very much aware of the currentUsually, you have to calculate the theoretical yield based on the balanced equation In this equation, the reactant and the product have a 11 mole ratio, so if you know the amount of reactant, you know the theoretical yield is the same value in moles (not grams!)You take the number of grams of reactant you have, convert it to moles, and then use this number of moles to find out how many


Extent Of Reaction For Material Balances Youtube


Stoichiometry And Percent Yield Examples Solutions Worksheets Videos Games Activities
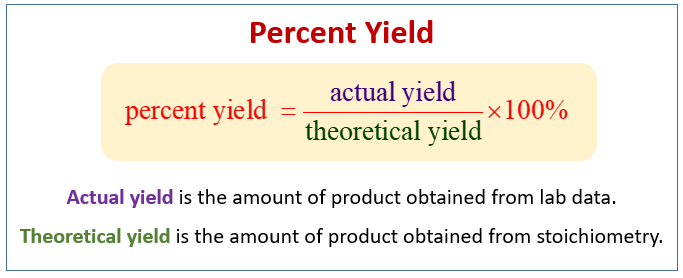
Prof Manolito E Bambase Jr Department of Chemical Engineering University of the Philippines Los Baños SLIDE 2 What can we learn from a chemical equation?C 7 H 16 11 O 2 7 CO 2 8 H 2 O 1 What information can we get from this equation?Percent yield or percentage yield is the ratio of the actual yield and the theoretical yield of a chemical reaction The experimental yield is divided by the theoretical yield and multiplied by 100 to be calculated as the percent yield If the theoretical yield and the experimental yield are same then the percent yield will be 100%



Chemistry Step By Step Solutions Chemical Reactions Wolfram Blog


Q Tbn And9gctgqe Sq69j9qphav8vjnq7bdheoratbvanabkki0b6yruaya Usqp Cau
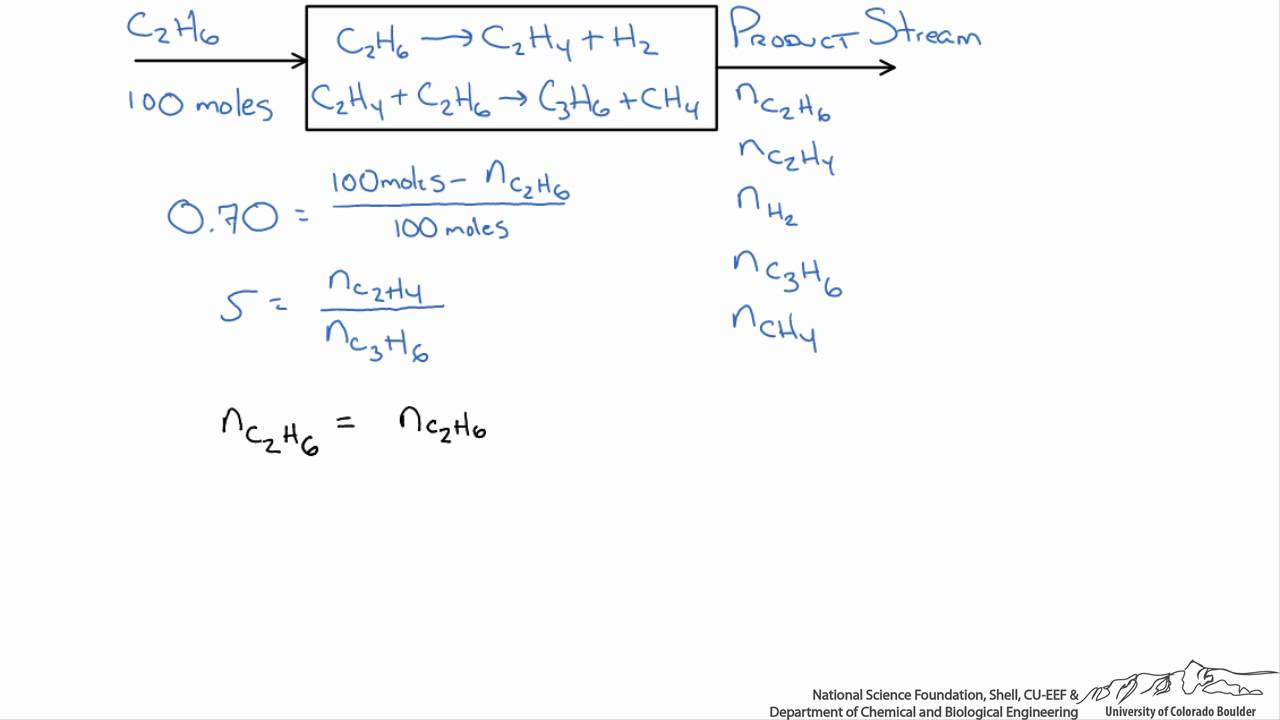
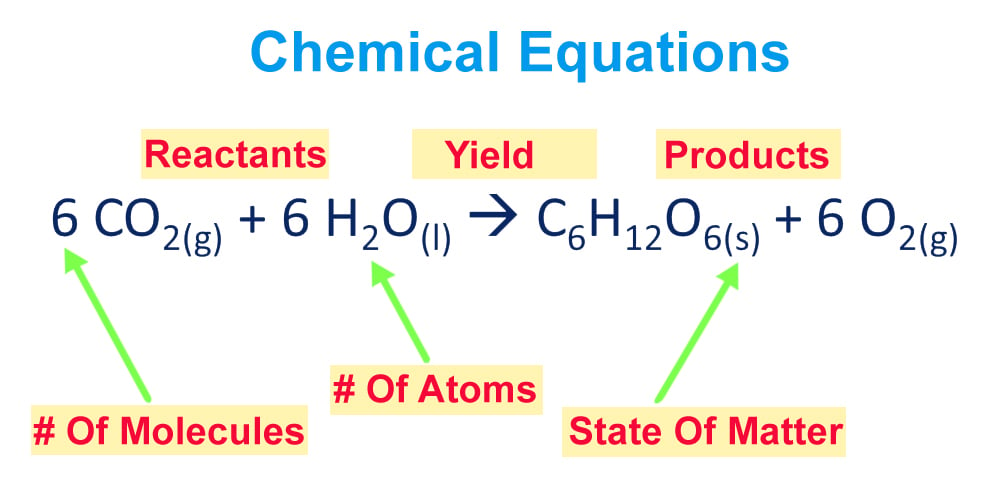
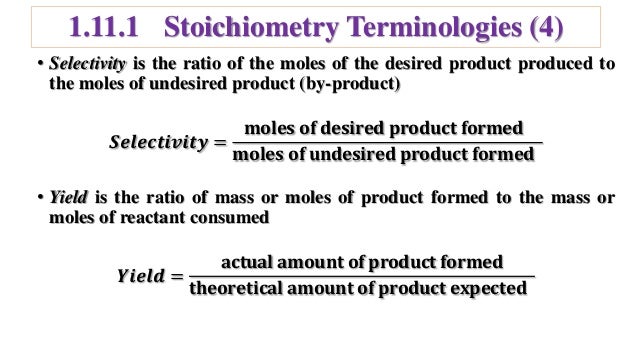
As the chemical engineering community is collecting more data (volume) from different sources (variety), this journey becomes more challenging in terms of using the right data and the right toolsIn the chemical literature (in particular the one devoted to engineering topics) it is necessary to use the terms of conversion (X), selectivity (S) and yield (Y) due to their relevance in kinetic studies, in order to adequately dimension reactors, to choose the best conditions to maximize the production of a given compound, limiting the amount ofThe following chemical reaction takes place ∑ i = 1 n ν i A i = ∑ j = 1 m μ j B j {\displaystyle \sum _{i=1}^{n}\nu _{i}A_{i}=\sum _{j=1}^{m}\mu _{j}B_{j}} , where ν i {\displaystyle \nu _{i}} and μ j {\displaystyle \mu _{j}} are the stoichiometric coefficients



Chemical Equations Protocol



Reaction Yield An Overview Sciencedirect Topics
INTEGRATED CIRCUIT ENGINEERING CORPORATION 31 3 Yield and Yield Management Product Metric Best Score Average Score Worst Score Memory CMOS Logic MSI Line Yield Die Yield Line Yield Die Yield Line Yield Die Yield 9 936 972 786 912 567 930 774 8 711 779 495 871 528 778 486 659 431 * 2Q mask layers, ~1m feature size, 05sqSo the only thing you need to do is to plug these values in the percent yield formula The calculation of the percent yield in chemical reactions is very importantDepartment of Chemical Engineering Yield coefficients, Y, are defined based on the amount of "Bioprocess Engineering Basic Concepts, Shuler and Kargi, Prentice Hall, 02 32 David R Shonnard Michigan Technological University Batch Growth Data and Monod Parameters



How To Calculate Percent Yield In Chemistry 15 Steps



Calculating Reaction Yield And Percentage Yield From A Limiting Reactant Science Class Video Study Com
Yield variance is the difference between actual output and standard output of a production or manufacturing process, based on standard inputs of materials and laborWithin these industries, chemical engineers rely on their knowledge of mathematics and science—particularly chemistry— to overcome technical problems safely and economically And, of course, they draw upon and apply their engineering knowledge to solve any technical challenges they encounterChemical engineering design GAVIN TOWLER, RAY SINNOTTpdf Nitin Prajapat Download PDF
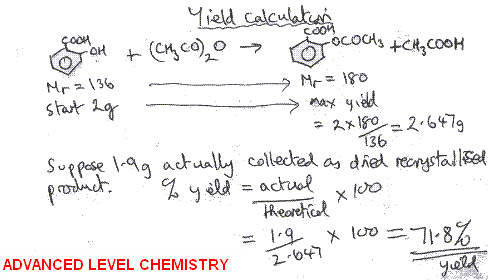


Howto How To Find Percent Yield Without Actual Yield


Chapter 6 Summary Notes
Many problems have a natural sequence, but for some, you may need to find one or more starting values that yield a desired final result 8/7 in the series Excel Tips for Chemical Engineers Excel Tip #5 Take Advantage of Data Tables for Case StudiesEngineering & Science This is the amount you would actually measure at the end of a chemical reaction in the lab Actual yield is the measured amount Determining the Chemical Formula FromProf Manolito E Bambase Jr Department of Chemical Engineering University of the Philippines Los Baños SLIDE 5 Example 111 Theoretical and Stoichiometric Air In a given process, 100 kmol of carbon is burned in a furnace It has been found that % of the carbon undergoes incomplete combustion resulting to CO production
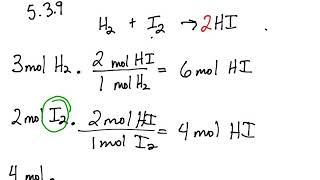


5 3 Calculating Reaction Yields Problems Chemistry Libretexts



Engineering Formula Sheet Engineering Notes Civil Engineering Handbook Engineering
Chris DeArmitt, in Applied Plastics Engineering Handbook, 11 2627 Yield Strength Yield strength is another important property that can be enhanced through the use of fillers Similar to the trends seen for modulus, the higher the aspect ratio of the filler, the greater its ability to raise yield strengthRearrange the above formula to obtain theoretical yield formula Example 1 Determine the theoretical yield of the formation of geranyl formate from 375 g of geraniol A chemist making geranyl formate uses 375 g of starting material and collects 417g of purified product Percentage yield is given as 941% SolutionThe percent yield is the ratio of the actual yield to the theoretical yield, expressed as a percentage \\text{Percent Yield} = \frac{\text{Actual Yield}}{\text{Theoretical Yield}} \times 100\%\ Percent yield is very important in the manufacture of products Much time and money is spent improving the percent yield for chemical production



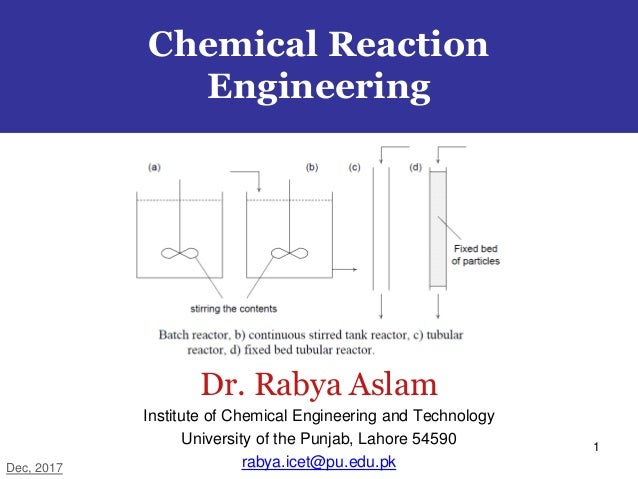
Batch Reactor An Overview Sciencedirect Topics


Balancing Chemical Equations How To Balance Chemical Equations
Yield variance is the difference between actual output and standard output of a production or manufacturing process, based on standard inputs of materials and laborTotal yield (%) = (yield 1 st react × yield 2 nd react × , etc ) × 100 For example, in a chemical transformation composed of three reactions and with partial yields each of 25%, 50%, and 75%, respectively, to calculate the total yield the above equation is applied (expressing the partial yields to decimals), resulting the following expressionMar 05, 21 Chemical engineering formulae GATE Notes EduRev is made by best teachers of GATE This document is highly rated by GATE students and has been viewed 2232 times


8 6 Limiting Reactant Theoretical Yield And Percent Yield From Initial Masses Of Reactants Chemistry Libretexts



Chapter 3 Calculation With Chemical Formulas And Equations Pdf Free Download
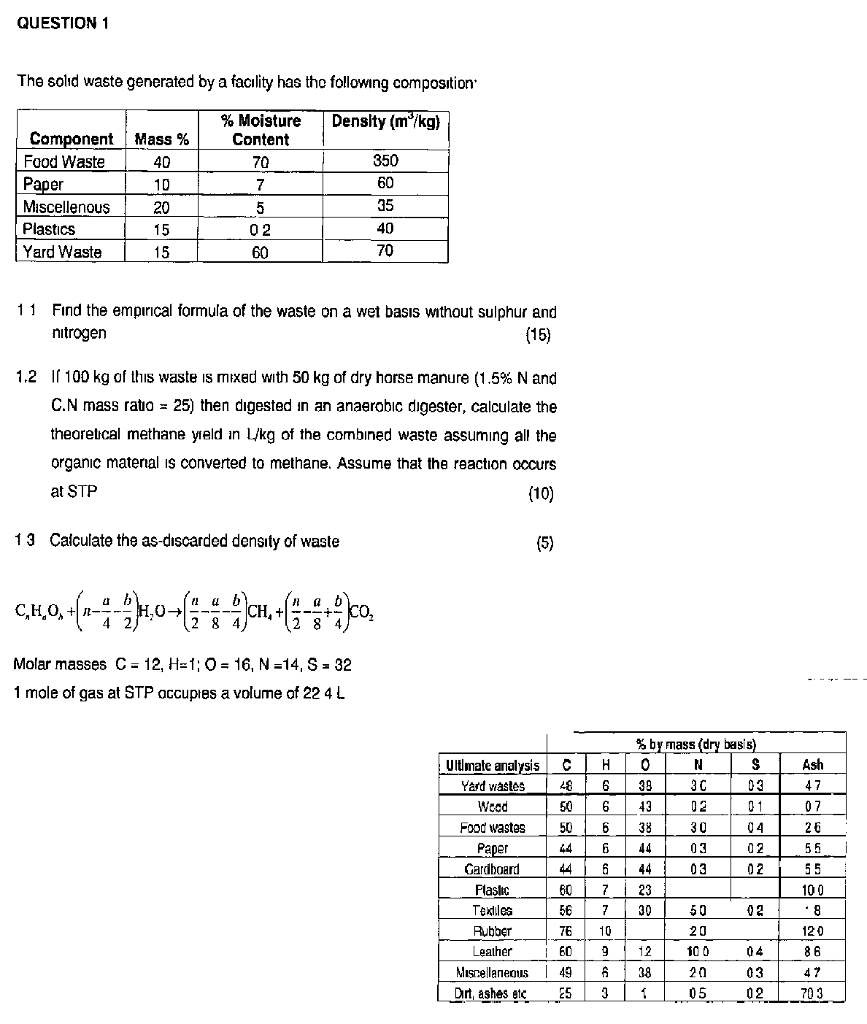
The fractional conversion of methane is 0900 and the fractional yield of formaldehyde is 0855 Calculate the molar composition of the reactor output stream and the selectivity of the formaldehyde production relative to carbon dioxide productionINTEGRATED CIRCUIT ENGINEERING CORPORATION 31 3 Yield and Yield Management Product Metric Best Score Average Score Worst Score Memory CMOS Logic MSI Line Yield Die Yield Line Yield Die Yield Line Yield Die Yield 9 936 972 786 912 567 930 774 8 711 779 495 871 528 778 486 659 431 * 2Q mask layers, ~1m feature size, 05sqPLTW, Inc Engineering Formulas y footing A = area of foot Structural Design qnet Steel Beam Design Moment M n = F y Z x M a = allowable bending moment M n = nominal moment strength Ω b = 167 = factor of safety for bending moment F y = yield stress Z x = plastic section modulus about neutral axis Spread Footing Design = q allowable p footing q


Chemical Reaction Engineering



Solved Chemistry For Engineering 1421 105 Q1 How Many Gr Chegg Com
Niques used in the field of chemical engineering as well as biological, petroleum, and environmental engineering Although the range of subjects deemed to be in the province of chemical engineering has broadened over the last twenty years, the basic principles of this field of study remain the sameThis simple estimation will often yield a result greatly under a value needed for a good cost estimations because in the modern financial economy, cash deposits yield minimal interest rates WACC is described by the following formula 3 where is the number of sources of capital Chemical Engineering Design Principles, Practice andHigher yield Depending on feed purity, product purity specification, and the extent of overlap of product and impurities, a 40%–90% higher yield can be obtained without compromising target purity Higher productivity Yield improvement and product overloading without process performance decline leads to higher productivity



Calculating Reaction Yield And Percentage Yield From A Limiting Reactant Science Class Video Study Com


Introduction To Engineering Calculations Bio Engineering
GATE Study Material for Chemical Engineering This is Chemical Engineering study material for GATE / PSUs exam preparation in the form of handwritten notes Candidates may refer this study material for their GATE / PSUs and other National & State level exam preparation Candidates can download these notes from the table given belowHigher yield Depending on feed purity, product purity specification, and the extent of overlap of product and impurities, a 40%–90% higher yield can be obtained without compromising target purity Higher productivity Yield improvement and product overloading without process performance decline leads to higher productivityIn chemistry, yield (also called chemical yield or reaction yield) is the amount of product resulting from a chemical reaction The absolute yield gives the weight in grams, and the molar yield gives the number of moles The fractional yield, relative yield, or percentage yield shows how completely a synthetic procedure worked It is calculated by dividing the amount of product by the


Howto How To Find Percent Yield Without Actual Yield



Reaction Yield Protocol
The theoretical molar yield is mol (the molar amount of the limiting compound, acetic acid) The molar yield of the product is calculated from its weight (132 g ÷ g/mol = 15 mol) The % yield is calculated from the actual molar yield and the theoretical molar yield (15 mol ÷ mol × 100% = 75%) citation needed(A) 159 (B) 50 (C) 632 (D) 100 63 The characteristics equation of closed loop system is 3 2 6 22 6 (1) 0 s s s KDefinition of Conversion, Selectivity and Yield This is the definition that we used in our simulation work



Depaktment Of Chemical Engineering The City Colleg Chegg Com


Ceng Tu Edu Iq Ched Images Lectures Chem Lec St1 C3 Basic 1 Pdf
Prof Manolito E Bambase Jr Department of Chemical Engineering University of the Philippines Los Baños SLIDE 2 What can we learn from a chemical equation?Chemists have to be concerned with just how completely their reactants react to form products To compare the amount of product obtained from a reaction with the amount that should have been obtained, they use percent yield You determine percent yield of a chemical reaction with the following formula Lovely, but what is an actual


Chapter 6 Summary Notes



Chemical Reaction Engineering Asynchronous Video Series Chapter 6 Part 1 Series Parallel And Complex Liquid Phase Reactions Selectivity Mole Balances Ppt Download



Process Chemistry Wikipedia



Conversion Chemistry Wikipedia



How To Calculate Percent Yield In Chemistry 15 Steps



What Is Yield Stress Definition Formula Video Lesson Transcript Study Com



How To Calculate Percent Yield In Chemistry 15 Steps



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com


Unsteady Cstrs And Semibatch Reactors



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com



How To Calculate Percent Yield In Chemistry 15 Steps


Chemical Engineering Question Chegg Com



Calculation Methods Of Yield Strength And Ultimate Tensile Strength By Download Scientific Diagram



Howto How To Find Percent Yield Without Actual Yield



Chemical Engineering Calculation Chemical Engineering Calculations Stoichiometry Studocu



Chapter 3 Material Balance Part Ii Ppt Video Online Download



Chemistry Step By Step Solutions Chemical Reactions Wolfram Blog


Q Tbn And9gcqbx4lhvwffwq2jalu0eardaoc5r99ldcrwpwdghn9sl8ldwbrs Usqp Cau


The Best Way To Calculate Percent Yield In Chemistry Wikihow



Percent Yield Percent Purity Solutions Examples Videos



Howto How To Find Percent Yield Without Actual Yield



Plasticity Physics Wikipedia


Unsteady Cstrs And Semibatch Reactors
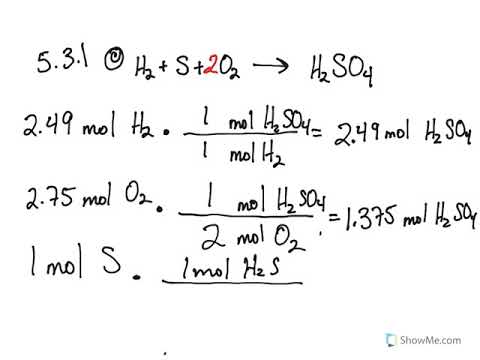


5 3 Calculating Reaction Yields Problems Chemistry Libretexts
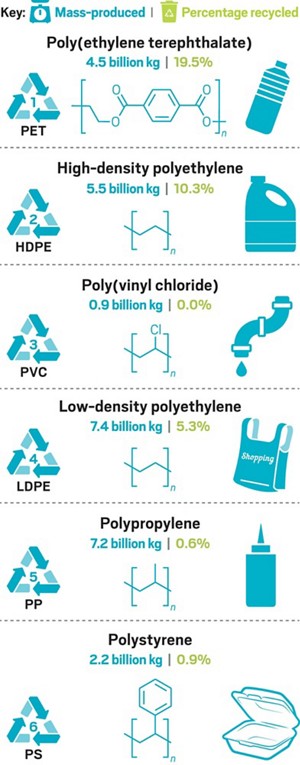


Chemistry May Have Solutions To Our Plastic Trash Problem



Chemical Reactions And Chemical Equations Owlcation Education



What Is Yield Stress Definition Formula Video Lesson Transcript Study Com



Two Reactions Extent Of Reaction Method Youtube



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com


Core Ac Uk Download Pdf Pdf
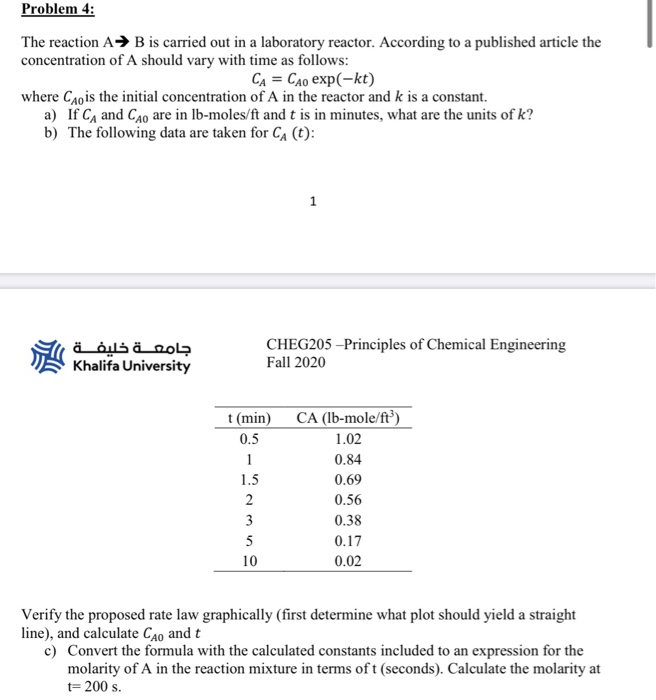


Solved Problem 4 The Reaction A B Is Carried Out In A L Chegg Com



Department Of Chemical Engineering Review Sheet Chemical Reactions Prepared By Dr Timothy D Placek From Various Sources Pdf Free Download



How To Calculate Percent Yield In Chemistry 15 Steps
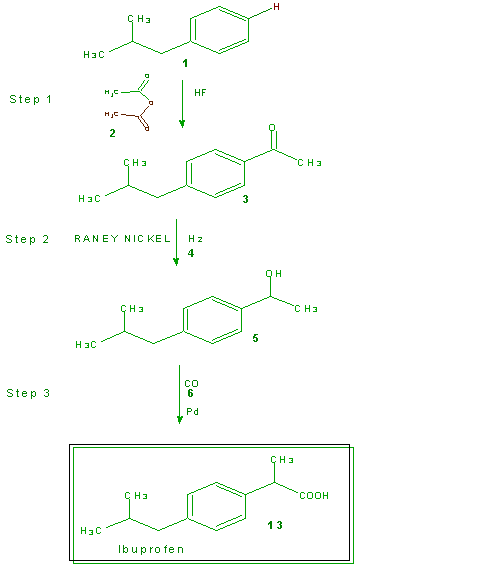


Green Chemistry English Green Chemistry



Howto How To Find Percent Yield Without Actual Yield


Rmp Lecture Notes



Ptt108 108 Material And Energy Balance Ppt Download



Calculating Reaction Yield And Percentage Yield From A Limiting Reactant Science Class Video Study Com
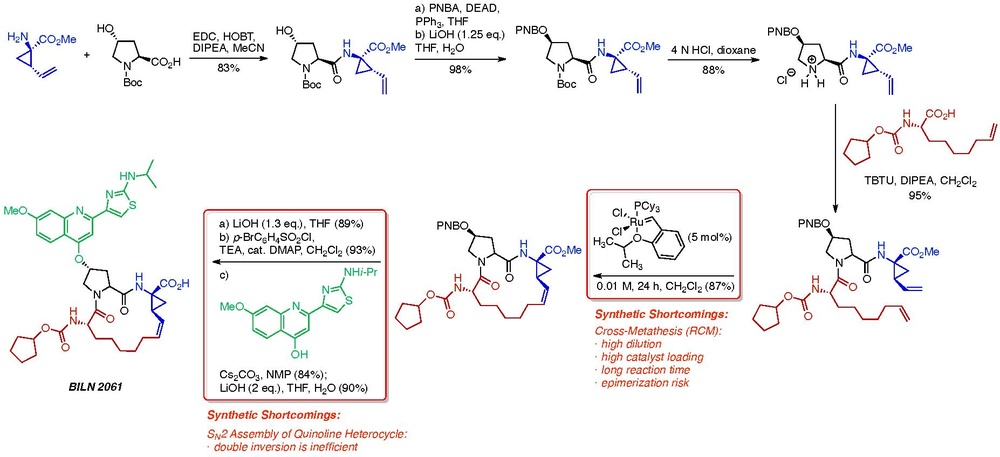


Process Chemistry Wikipedia
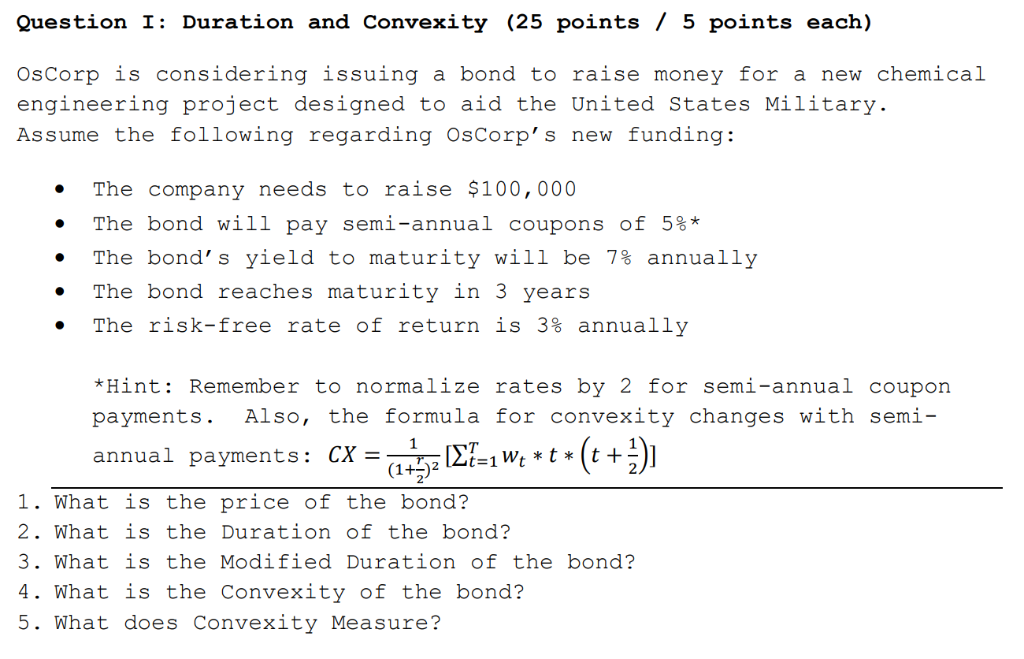


Question I Duration And Convexity 25 Points5 Poi Chegg Com
/148302528-56a12f323df78cf77268383a.jpg)


Percent Yield Definition And Formula
/two-hands-pour-contents-of-test-tube-into-laboratory-flask-155006089-5940123e5f9b58d58a12508a.jpg)


Overview Of Excess Reactant In Chemistry



How To Calculate Percent Yield In Chemistry 15 Steps
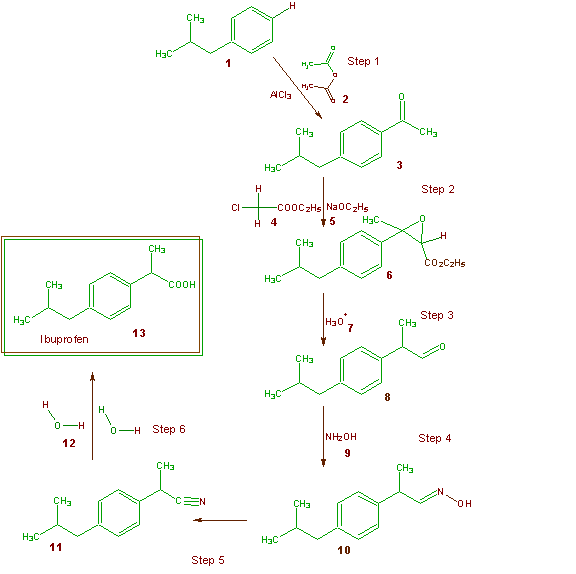


Green Chemistry English Green Chemistry



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com



Calculating Reaction Yield And Percentage Yield From A Limiting Reactant Science Class Video Study Com


Ceng Tu Edu Iq Ched Images Lectures Chem Lec St1 C3 Basic 1 Pdf
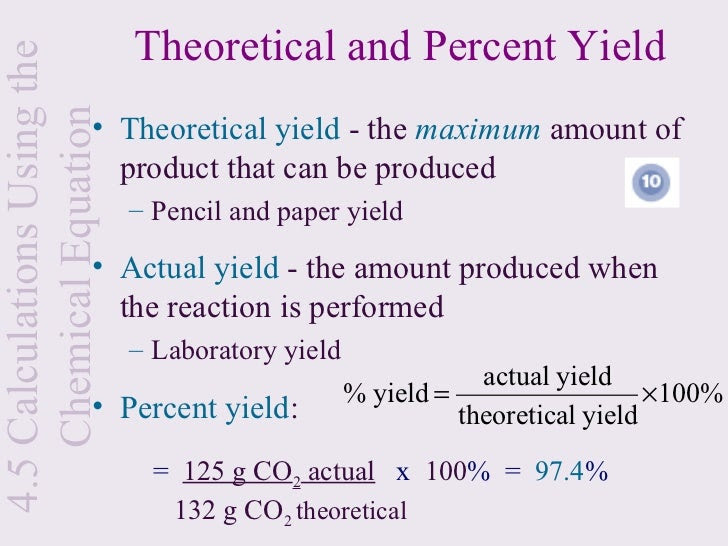


Howto How To Find Percent Yield Without Actual Yield
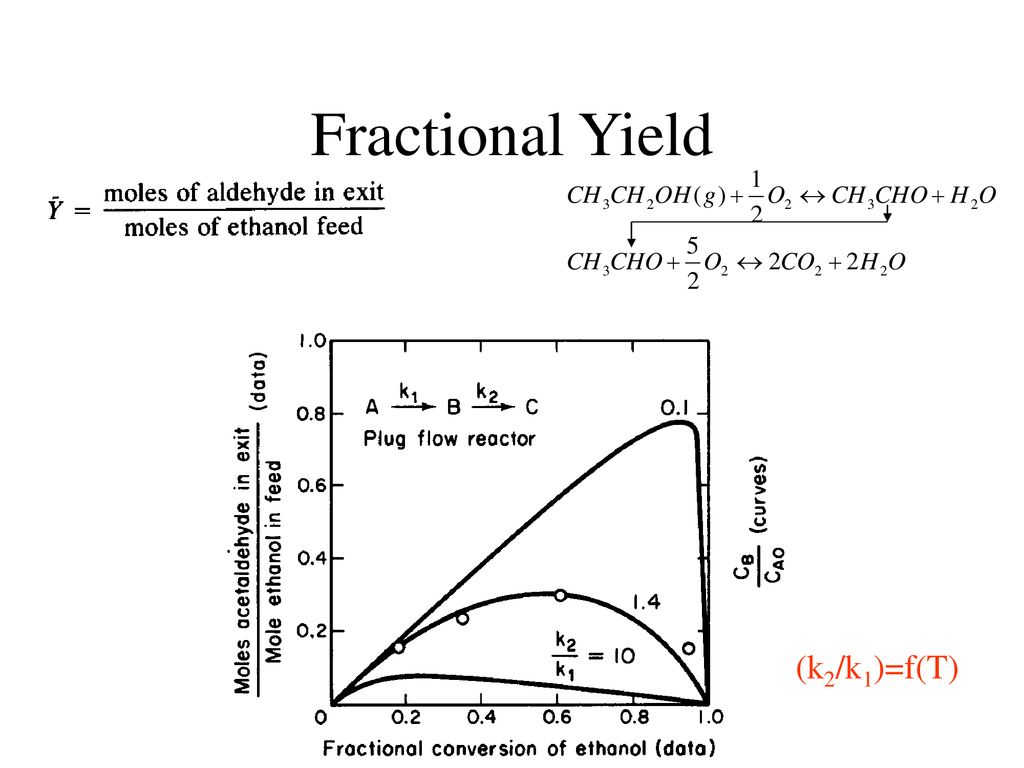


Reactor Design For Selective Product Distribution Ppt Download
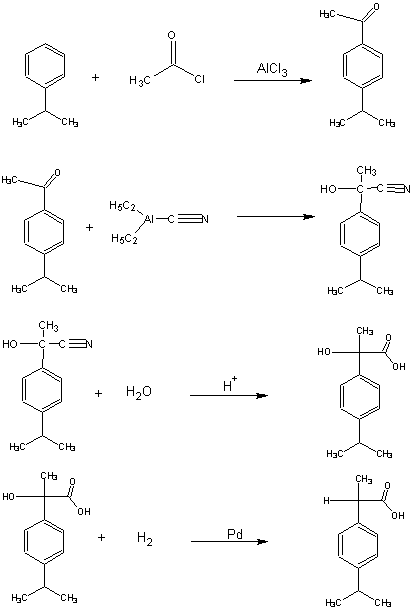


Green Chemistry English Green Chemistry



Reaction Yield An Overview Sciencedirect Topics



Howto How To Find Percent Yield Without Actual Yield



Chemistry Review Of Stoichiometry Oli Chemistry Chemical Science Chemistry Review



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com



Percent Yield Calculator For Chemistry Equations
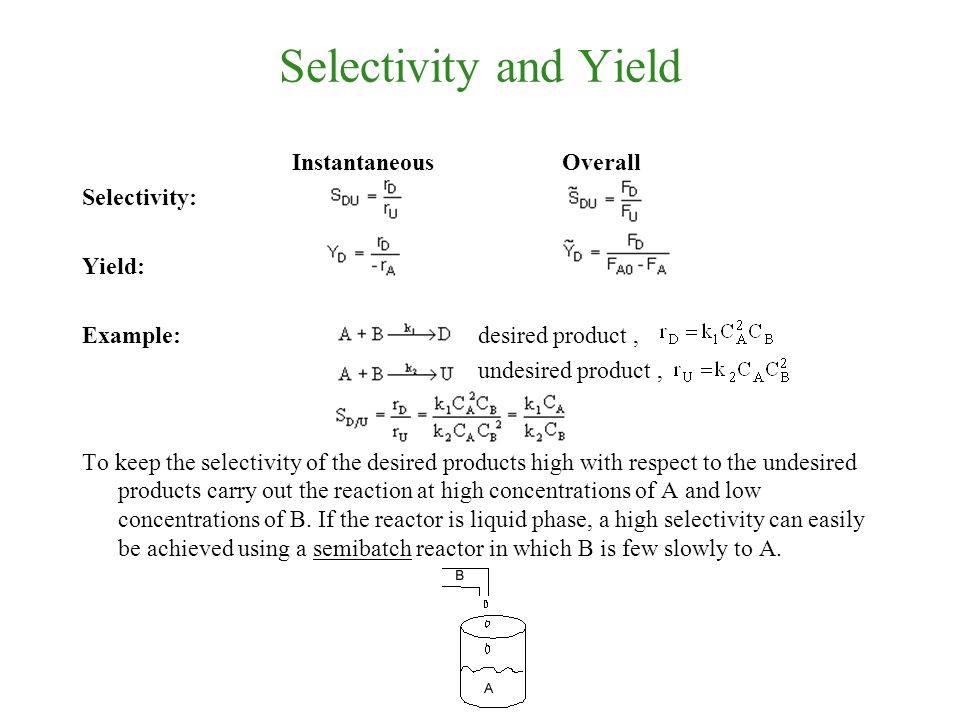


Chemical Reaction Engineering Asynchronous Video Series Ppt Video Online Download


3



Percent Yield Percent Purity Solutions Examples Videos


Http Faculty Poly Edu Rlevicky Handout3 Pdf


Http Faculty Poly Edu Rlevicky Handout3 Pdf



Reaction Yield An Overview Sciencedirect Topics
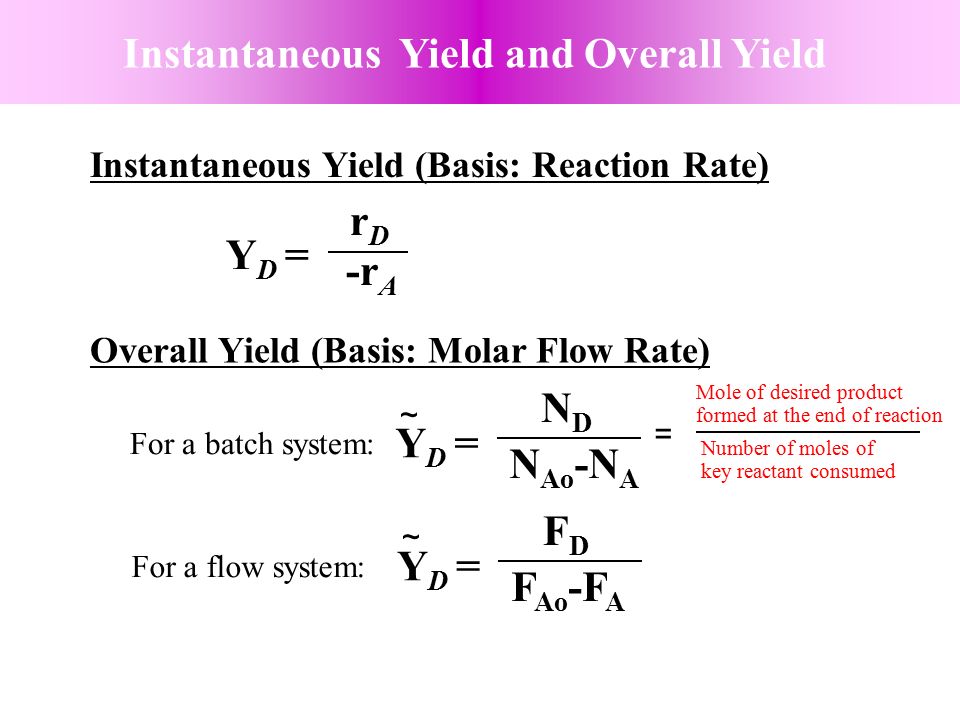


Chapter 6 Chemical Reaction Engineering Mutiple Reactions Ppt Video Online Download


Mass Relationships In Chemical Equations



The Bioprocess Tea Calculator An Online Techno Economic Analysis Tool To Evaluate The Commercial Competitiveness Of Potential Bioprocesses Biorxiv


Chapter 6 Summary Notes



Reaction Yield Protocol


Chapter 6 Summary Notes


1



Selectivity In Series Reactions Youtube
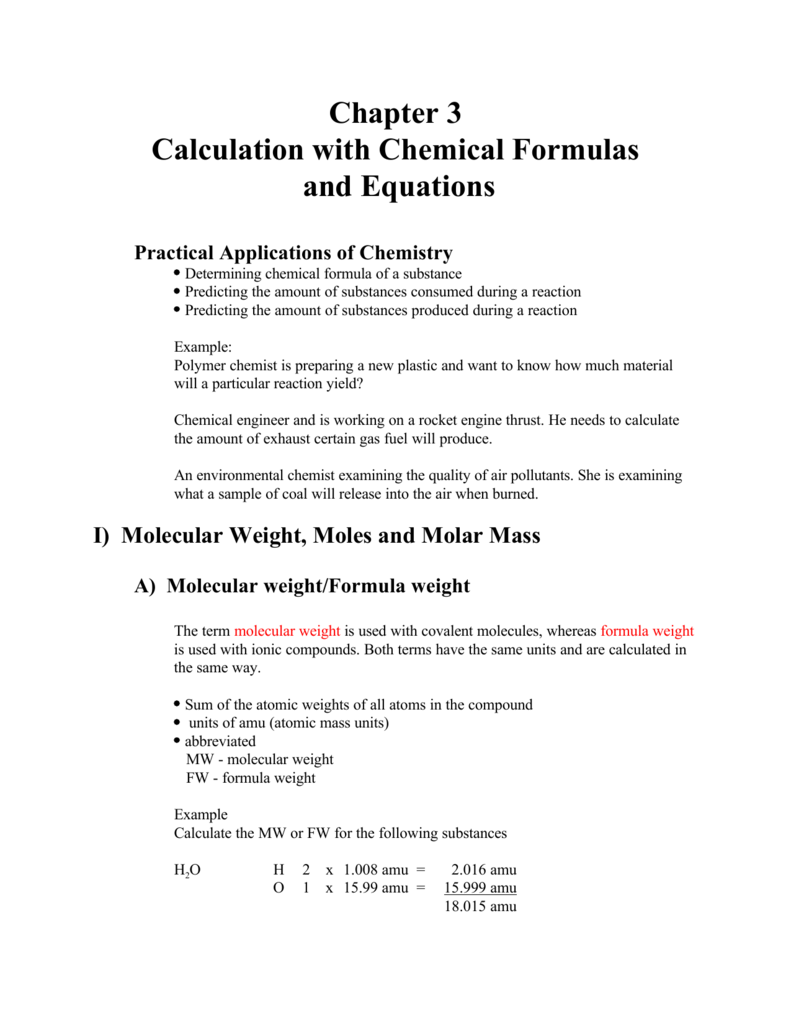


Chapter 3 Calculation With Chemical Formulas And Equations



Percent Yield Calculator For Chemistry Equations



Percent Yield Percent Purity Solutions Examples Videos



Percent Yield Calculator Find Percent Yield With Formula Equation
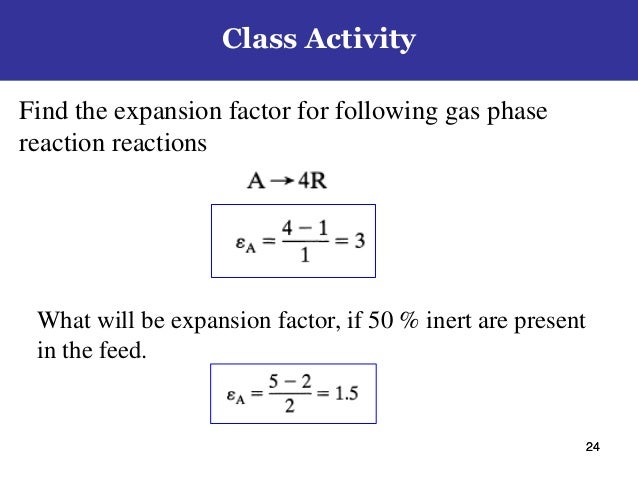


Chemical Reaction Engineering



Reaction Yield Protocol


The Pillars Curriculum For Chemical Engineering
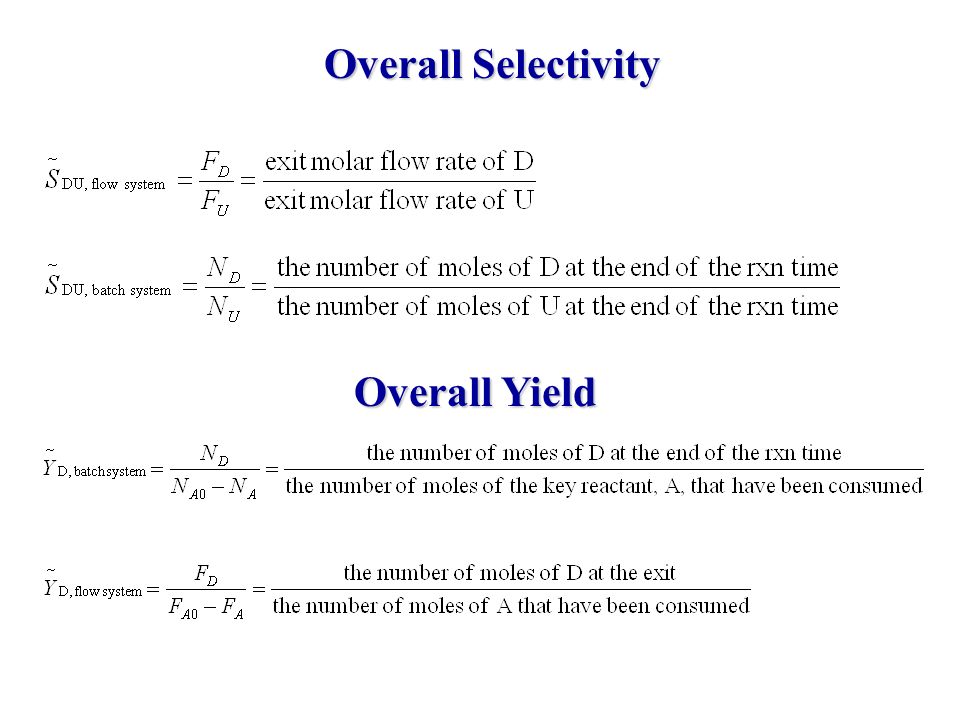


Chapter 6 Chemical Reaction Engineering Mutiple Reactions Ppt Video Online Download



C3a Working With Multiple Reactions Yield Selectivity Youtube


コメント
コメントを投稿